Krypton Periodic Table Protons Neutrons And Electrons

Its monatomic form h is the most abundant chemical substance in the universe constituting roughly 75 of all baryonic mass.
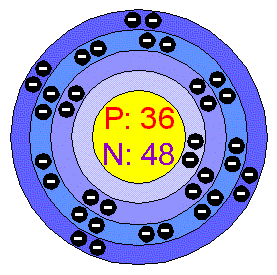
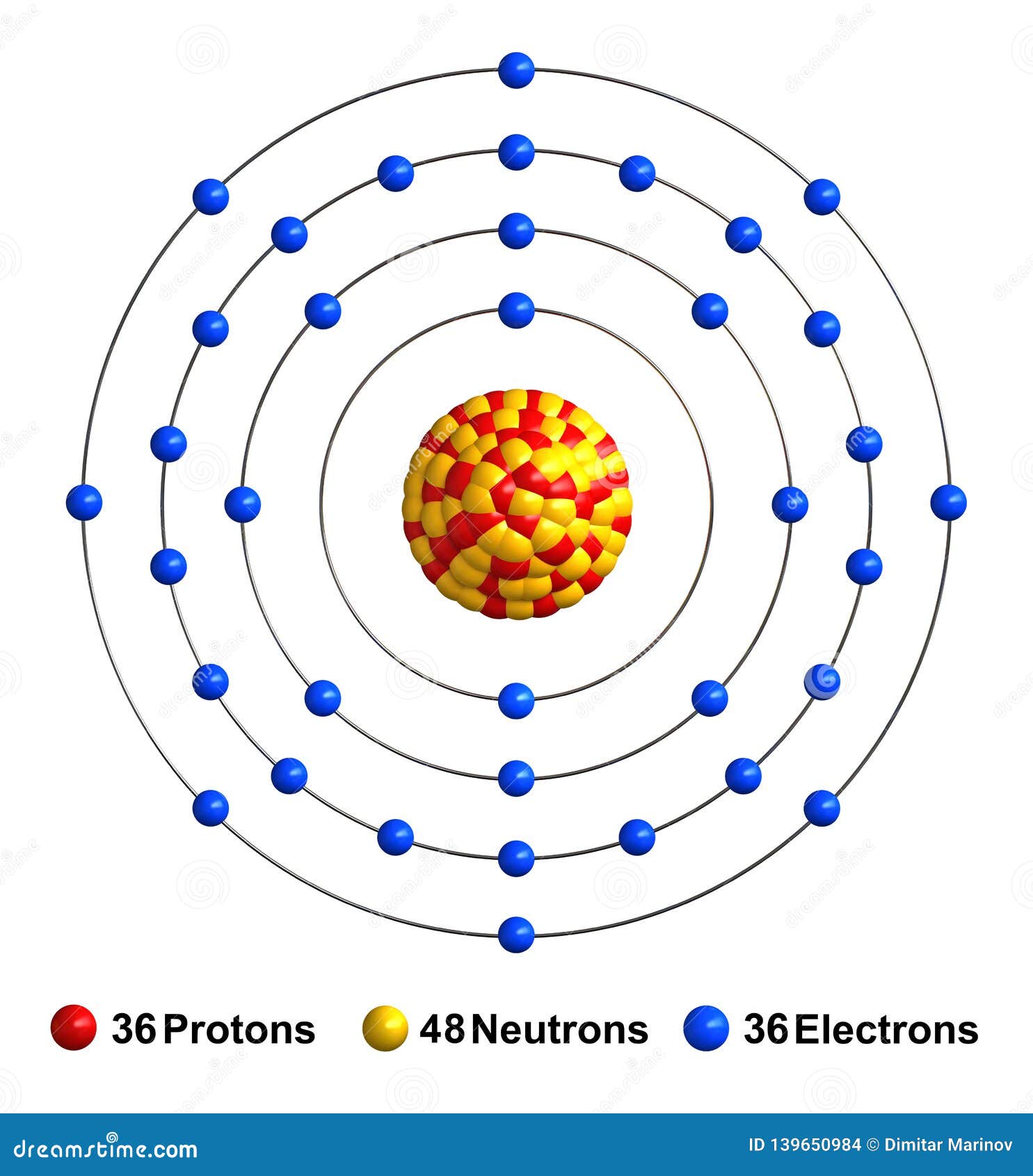
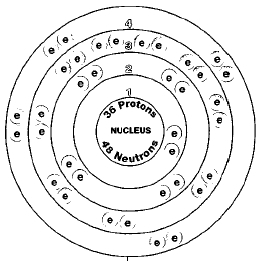
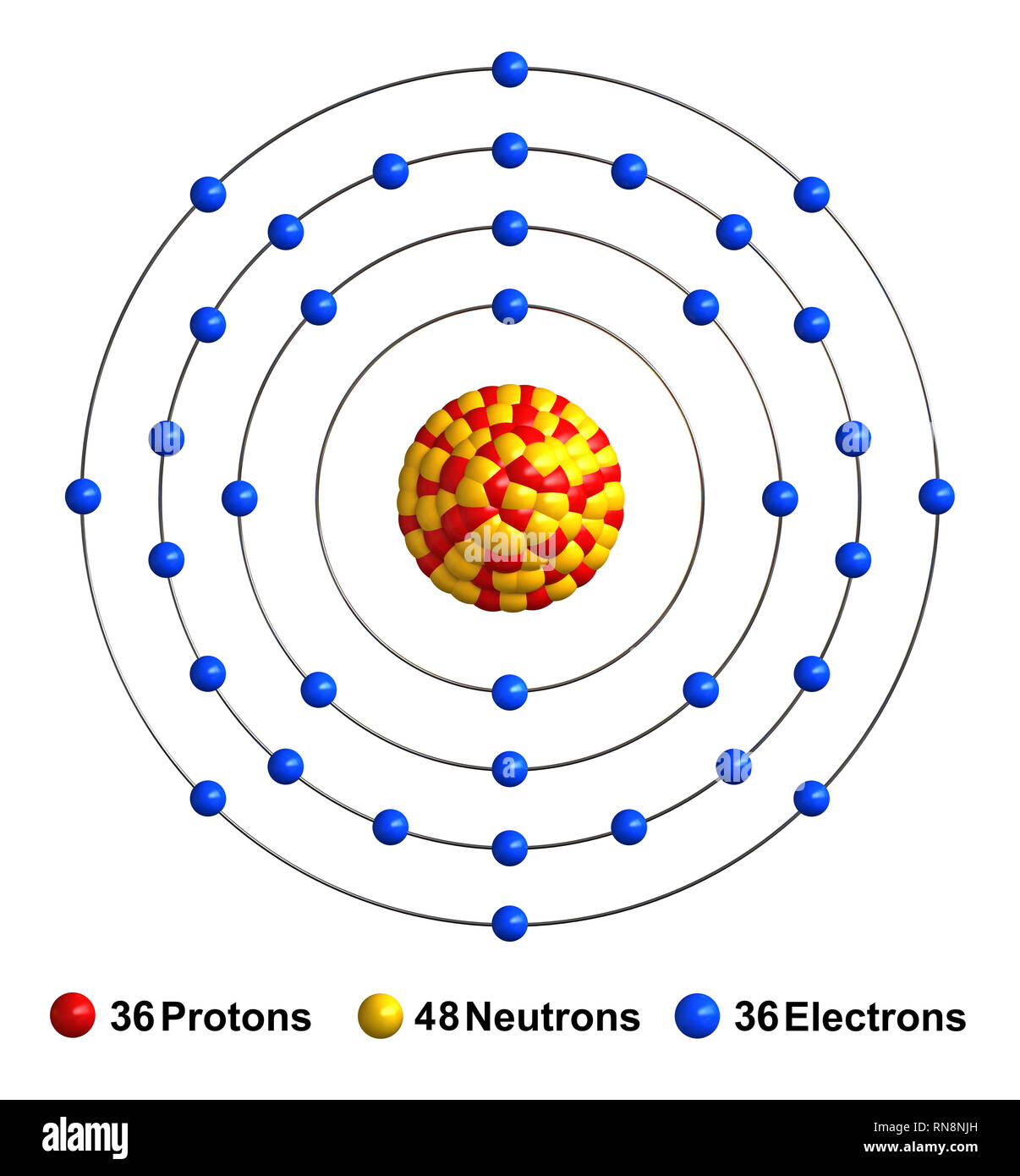
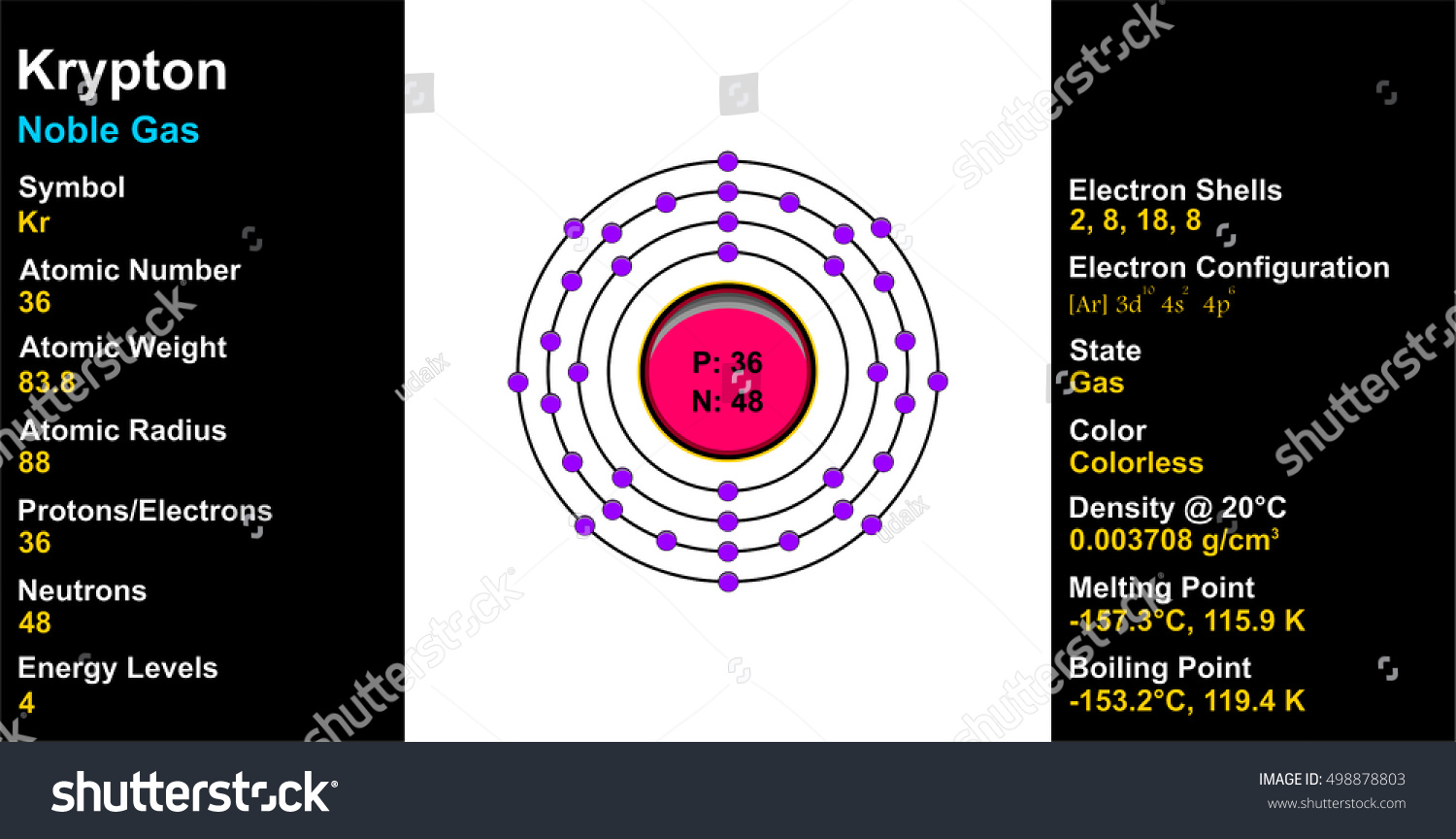
Krypton periodic table protons neutrons and electrons. No matter how many electrons or neutrons an atom has the element is defined by its number of protons. Hi nancy to get the mass number we add the number of protons and neutrons. A horizontal row in the periodic table. Whats people lookup in this blog.
It is found free in nature. 3 74 g cm 3 color. The number of protons is equal to the number of electrons unless there s an ion superscript listed after the element. Noble gas crystal structure.
83 8 amu melting point 157 2 c 115 950005 k 250 95999 f boiling point 153 4 c 119 75001 k 244 12 f number of protons electrons. Sharp s principal p diffuse d and fundamental f. Protons neutrons and electrons the relative mass relative charge and location in the atom for each subatomic particle is given in the table. Periodic table of elements activities create an atom diagram periodic table of elements list with protons neutrons and electrons first things chemistry project crystal how many protons neutrons and electrons does neon have quora periodic table of elements list with protons neutrons and electrons.
Colorless gas atomic structure. Krypton difluoride krf 2 interesting facts. Krypton s most abundant isotope has 48 neutrons so its mass number is 36 48 84. The periodic table is arranged in order of increasing atomic number so the number of protons is the element number.
It is produced when air is purified compressed cooled distilled and condensed. With a standard atomic weight of circa 1 008 hydrogen is the lightest element on the periodic table. In fact it s actually possible to have an atom consisting of only a proton ionized hydrogen. It is usually mixed with a halogen usually fluorine when used in lasers.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure the chemical symbol for hydrogen is h. Cubic density 293 k. The atomic number of each element increases by one reading from left to right. Block elements are organised into blocks by the orbital type in which the outer electrons are found.
With a standard atomic weight of circa 1 008 hydrogen is the lightest element on the periodic table. The easiest way to find the number of protons neutrons and electrons for an element is to look at the element s atomic number on the periodic table. These blocks are named for the characteristic spectra they produce. The next most abundant isotope is krypton 86 which has 50 neutrons and a mass number of 86.
That number is equal to the number of protons.